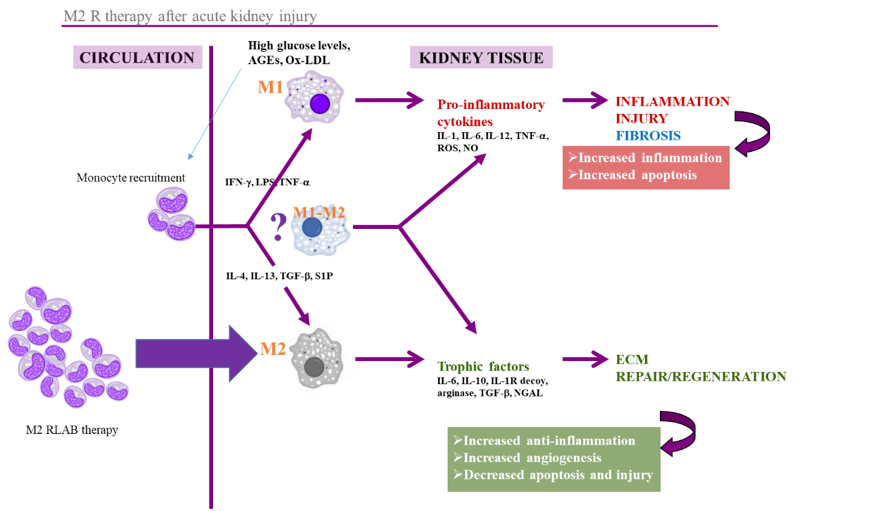
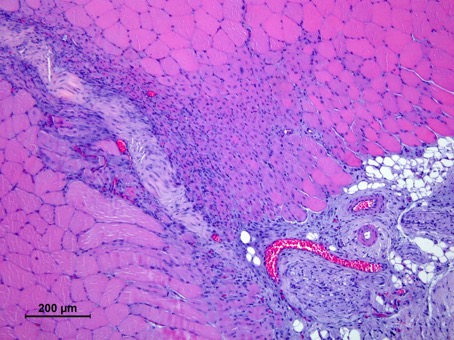
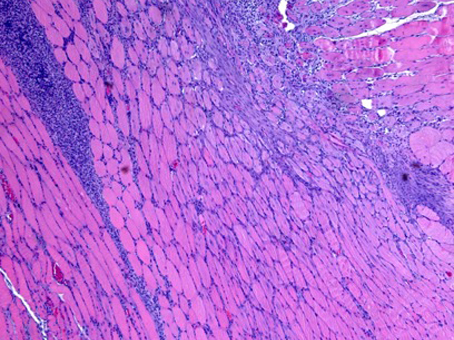
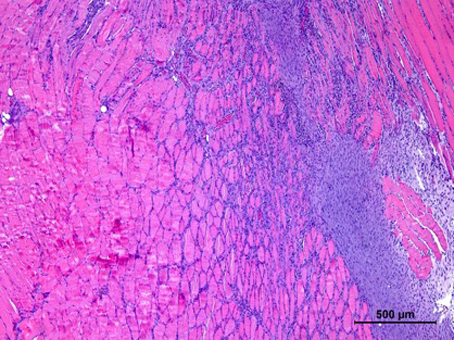
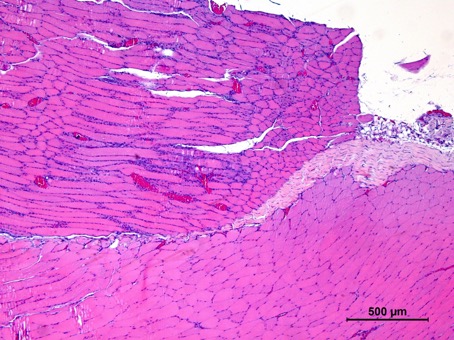
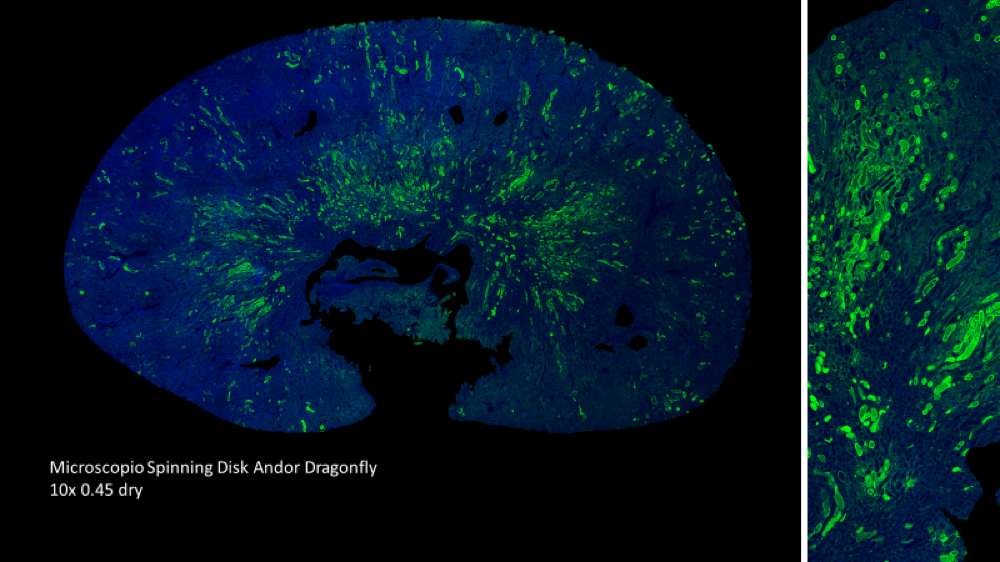
Our cell therapy, unlike stem cell therapies or grow factors, is based on the use of immune system cells, that act as a regulators of the regenerative process. Using our proprietary and patented M2R technology, we can increase the regenerative potential of the cells, promoting the expression of trophic factors that regulate the inflammation, as well as the proliferation and differentiation of the cell in the injury area.
The regenerative capacity of the M2R therapy has been validated in animal models
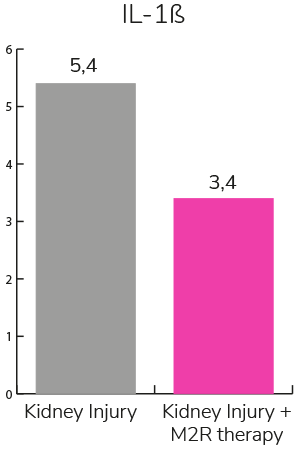
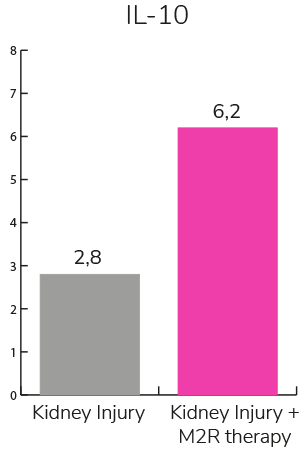
- Reduces inflammation and fibrosis
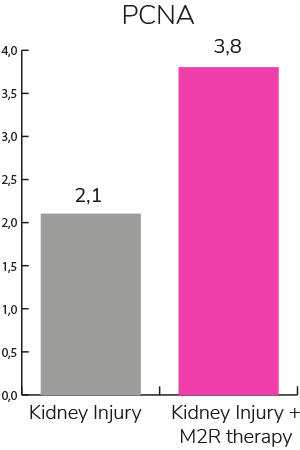
- Favors proliferation and differentiation of stem cell in the injury area